The regulatory domain of the RIG-I family ATPase LGP2 senses double-stranded RNA
10-Feb-2009
Nucleic Acids Res, 2009, doi:10.1093/nar/gkp059, 1-12 published on 10.02.2009
Nucleic Acids Research, online article
Nucleic Acids Research, online article
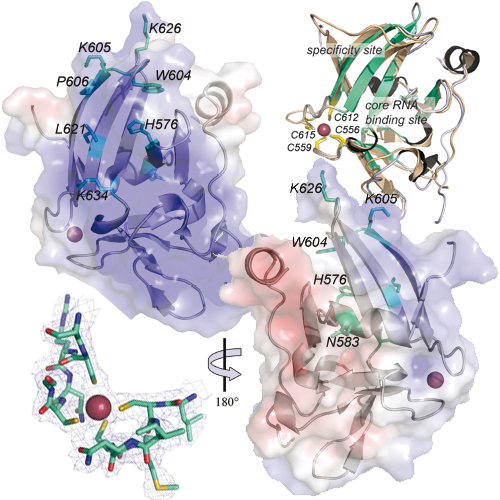
RIG-I and MDA5 sense cytoplasmic viral RNA and set-off a signal transduction cascade, leading to antiviral innate immune response. The third RIG-Ilike receptor, LGP2, differentially regulates RIG-Iand MDA5-dependent RNA sensing in an unknown manner. All three receptors possess a C-terminal regulatory domain (RD), which in the case of RIG-I senses the viral pattern 5’-triphosphate RNA and activates ATP-dependent signaling by RIG-I. Here we report the 2.6A ° crystal structure of LGP2 RD along with in vitro and in vivo functional analyses and a homology model of MDA5 RD. Although LGP2 RD is structurally related to RIG-I RD, we find it rather binds double-stranded RNA (dsRNA) and this binding is independent of 5’-triphosphates. We identify conserved and receptor-specific parts of the RNA binding site. Latter are required for specific dsRNA binding by LGP2 RD and could confer pattern selectivity between RIG-I-like receptors. Our data furthermore suggest that LGP2 RD modulates RIG-I-dependent signaling via competition for dsRNA, another pattern sensed by RIG-I, while a fully functional LGP2 is required to augment MDA5-dependent signaling.