Regulated Intramembrane Proteolysis of Bri2 (Itm2b) by ADAM10 and SPPL2a/b
25-Oct-2007
J. Biol. Chem., 2007, doi/10.1074/jbc.M706661200, published on 25.10.2007
http://www.jbc.org, online article
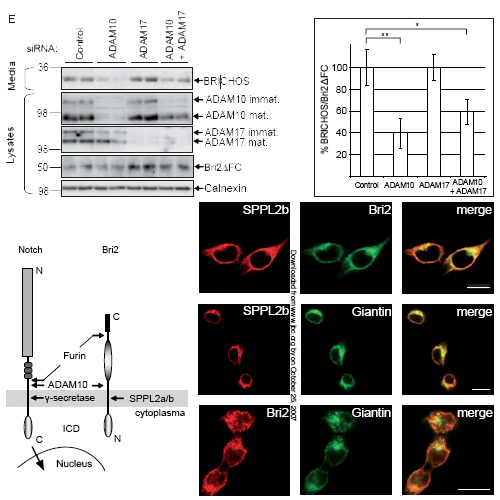
Presenilin, the catalytic component of the gamma-secretase complex, type IV prepilin peptidases and signal peptide peptidase (SPP) are the founding members of the family of intramembrane cleaving GxGD aspartyl proteases. SPP-like (SPPL) proteases, such as SPPL2a, b, and c and SPPL3 also belong to the GxGD family. In contrast to gamma-secretase, where numerous substrates have been identified, very few in vivo substrates are known for SPP and SPPLs. Here we demonstrate that Bri2 (Itm2b), a type-II oriented transmembrane protein associated with familial British and Danish dementia undergoes regulated intramembrane proteolysis (RIP). In addition to the previously described ectodomain processing by furin and related proteases, we now describe that the Bri2 protein, similar to gamma- secretase substrates, undergoes an additional cleavage by ADAM10 in its ectodomain. This cleavage releases a soluble variant of Bri2, the BRICHOS domain, which is secreted into the extracellular space. Upon this shedding event a membrane bound N-terminal Bri2 fragment (NTF) remains, which undergoes intramembrane proteolysis to produce an intracellular domain (ICD) as well as a secreted low molecular weight C-terminal peptide. By expressing all known SPP/SPPL family members as well as their loss of function variants, we demonstrate that selectively SPPL2a and SPPL2b mediate the intramembrane cleavage, while neither SPP nor SPPL3 are capable of processing the Bri2 NTF.