Mitochondrial Alkbh1 localizes to mtRNA granules and its knockdown induces the mitochondrial UPR in humans and C. elegans
01-Oct-2019
Journal of Cell Science; 2019; 132: jcs223891; doi: 10.1242/jcs.223891
Journal of Cell Science, online article
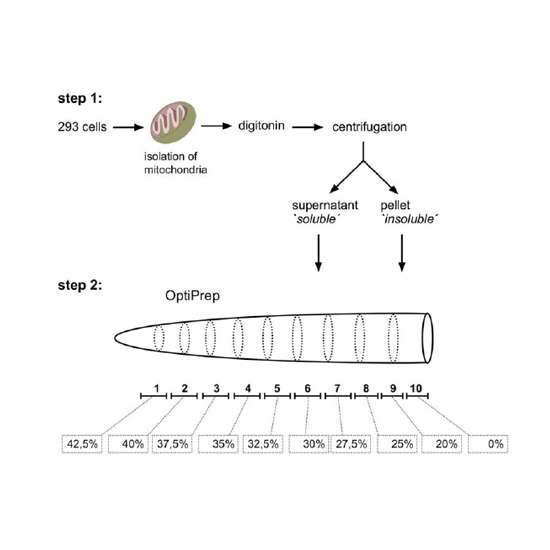
The Fe(II) and 2-oxoglutarate-dependent oxygenase Alkb homologue 1 (Alkbh1) has been shown to act on a wide range of substrates, like DNA, tRNA and histones. Thereby different enzymatic activities have been identified including, among others, demethylation of N3-methylcytosine (m3C) in RNA- and single-stranded DNA oligonucleotides, demethylation of N1-methyladenosine (m1A) in tRNA or formation of 5-formyl cytosine (f5C) in tRNA. In accordance with the different substrates, Alkbh1 has also been proposed to reside in distinct cellular compartments in human and mouse cells, including the nucleus, cytoplasm and mitochondria. Here, we describe further evidence for a role of human Alkbh1 in regulation of mitochondrial protein biogenesis, including visualizing localization of Alkbh1 into mitochondrial RNA granules with super-resolution 3D SIM microscopy. Electron microscopy and high-resolution respirometry analyses revealed an impact of Alkbh1 level on mitochondrial respiration, but not on mitochondrial structure. Downregulation of Alkbh1 impacts cell growth in HeLa cells and delays development in Caenorhabditis elegans, where the mitochondrial role of Alkbh1 seems to be conserved. Alkbh1 knockdown, but not Alkbh7 knockdown, triggers the mitochondrial unfolded protein response (UPRmt) in C. elegans.