Total Synthesis of Exiguamines A and B Inspired by Catecholamine Chemistry
13-Mar-2012
Chemistry - A European Journal, 2012, DOI: 10.1002/chem.201103605, Volume 18, Issue 16, pages 4999–5005 published on 13.03.2012
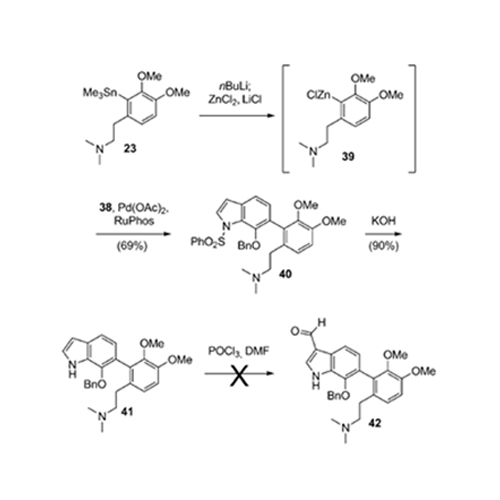
The evolution of a total synthesis of the exiguamines, two structurally unusual natural products that are highly active inhibitors of indolamine-2,3-dioxygenase (IDO), is described. The ultimately successful strategy involves advanced cross-coupling methodology and features a potentially biosynthetic tautomerization/ electrocyclization cascade reaction that forms two heterocycles and installs a quaternary ammonium ion in a single synthetic operation.