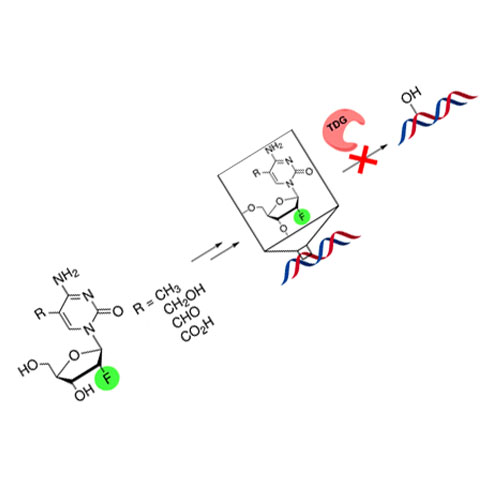
Synthesis of (R)-Configured 2′-Fluorinated mC, hmC, fC, and caC Phosphoramidites and Oligonucleotides
19-Aug-2016
Org. Lett., DOI: 10.1021/acs.orglett.6b02110
Org. Lett., online article
Investigation of the function of the new epigenetic bases requires the development of stabilized analogues that are stable during base excision repair (BER). Here we report the synthesis of 2′-(R)-fluorinated versions of the phosphoramidites of 5-methylcytosine (mC), 5-hydroxymethylcytosine (hmC), 5-formylcytosine (fC), and 5-carboxycytosine (caC). For oligonucleotides containing 2′-(R)-F-fdC, we show that these compounds cannot be cleaved by the main BER enzyme thymine-DNA glycosylase (TDG).