Identification of potential substrate proteins for the periplasmic Escherichia coli chaperone Skp
08-Dec-2008
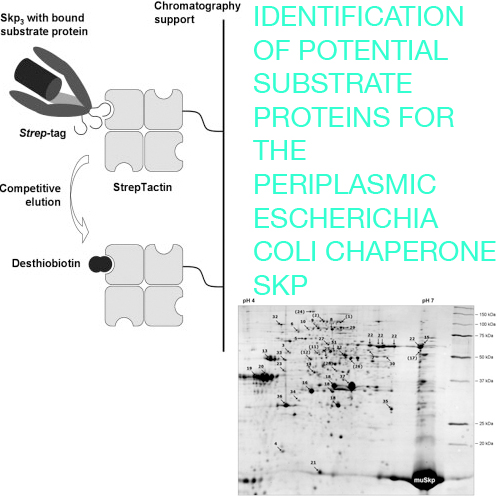
The “seventeen kilodalton protein” (Skp) is a predominant periplasmic chaperone of Escherichia coli, which is involved in the biogenesis of abundant outer membrane proteins (OMPs) such as OmpA, PhoE, and LamB. In this study the substrate profile of Skp was investigated in a proteomics approach. Skp was overexpressed in a deficient E. coli strain as a fusion protein with the Strep–tag and captured, together with any host proteins associated with it, from the periplasmic cell extract under mild conditions via one-step Strep–Tactin affinity chromatography. Copurified substrate proteins were then identified by high resolution 2-DE with immobilized pH-gradients, followed by MALDI-TOF MS. Apart from the known Skp substrates, including OmpA and LamB, more than 30 other interacting proteins were detected, especially from the outer membrane, among these FadL and BtuB, and from the periplasm such as MalE and OppA. Thus, Skp does not only serve as a specialized chaperone for a small set of OMPs, but it seems to exhibit a broader substrate spectrum, including soluble periplasmic proteins. These findings should prompt further investigation into the physiological role of Skp and may promote its use for the bacterial production of biochemically active heterologous proteins whose folding requires secretion into the oxidizing milieu of the periplasm.