Fusion of an alcohol dehydrogenase with an aminotransferase using a PAS linker to improve coupled enzymatic alcohol-to-amine conversion
29-Aug-2016
Protein Engineering, Design & Selection, vol. 29 no. 12, pp. 557–562, doi: 10.1093/protein/gzw039
Protein Engineering, Design & Selection, online article
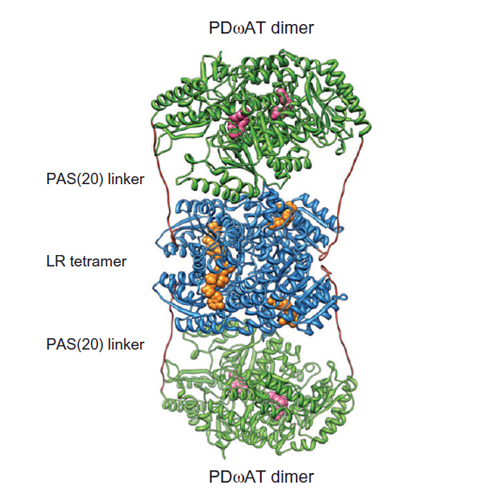
To facilitate biocatalytic conversion of the biotechnologically accessible dicyclic dialcohol isosorbide into its industrially relevant diamines, we have designed a fusion protein between two homo-oligomeric enzymes: the levodione reductase (LR) from Leifsonia aquatica and the variant L417M of the ω-aminotransferase from Paracoccus denitrificans (PDωAT(L417M)), mutually connected by a short Pro/Ala/Ser linker sequence. The hybrid protein was produced in Escherichia coli in correctly folded state, comprising a tetrameric LR moiety and presumably two dimers of PDωAT(L417M), as proven by SDS-PAGE and size exclusion chromatography. The bifunctional enzyme revealed beneficial kinetics over the two-component system, in particular at low substrate concentration.