Structures of Drosophila Cryptochrome and Mouse Cryptochrome1 Provide Insight into Circadian Function
06-Jun-2013
Cell, 2013, doi 10.1016/j.cell.2013.05.011, Volume 153, Issue 6, 1394-1405, published on 06.06.2013
Cell, online article
Cell, online article
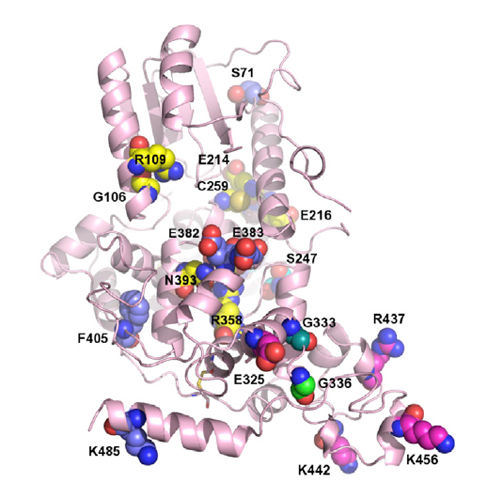
Drosophila cryptochrome (dCRY) is a FAD-dependent circadian photoreceptor, whereas mammalian cryptochromes (CRY1/2) are integral clock components that repress mCLOCK/mBMAL1-dependent transcription. We report crystal structures of full-length dCRY, a dCRY loop deletion construct, and the photolyase homology region of mouse CRY1 (mCRY1). Our dCRY structures depict Phe534 of the regulatory tail in the same location as the photolesion in DNA-repairing photolyases and reveal that the sulfur loop and tail residue Cys523 plays key roles in the dCRY photoreaction. Our mCRY1 structure visualizes previously characterized mutations, an NLS, and MAPK and AMPK phosphorylation sites. We show that the FAD and antenna chromophore-binding regions, a predicted coiled-coil helix, the C-terminal lid, and charged surfaces are involved in FAD-independent mPER2 and FBXL3 binding and mCLOCK/mBMAL1 transcriptional repression. The structure of a mammalian cryptochrome1 protein may catalyze the development of CRY chemical probes and the design of therapeutic metabolic modulators.