Vibralactone as a Tool to Study the Activity and Structure of the ClpP1P2 Complex from Listeria monocytogenes
11-Nov-2011
Angewandte Chemie, 2011, DOI: 10.1002/ange.201104391, Volume 50, Issue 46, pages 11001–11004 published on 11.11.2011
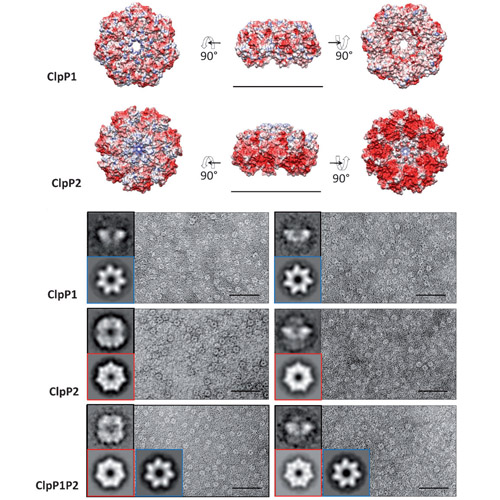
Nature provides a rich source of bioactive compounds comprising a diverse set of electrophilic core structures that are poised to react with corresponding nucleophilic residues such as serine and cysteine in enzyme active sites.These residues are usually relevant for catalysis and therefore display fine-tuned reactivity towards their dedicated substrates.We and others previously investigated the dedicated targets of monocyclic b-lactones which turned out to be potent and selective inhibitors of diverse disease-associated enzyme classes. Covalent inhibition of the caseinolytic peptidase ClpP, for instance, resulted in a dramatic attenuation of bacterial virulence. ClpP is an important, highly conserved heat shock protein with additional regulatory functions in many pathogens. Some organisms such as Listeria monocytogenes genetically encode for two functionally and structurally uncharacterized ClpP isoforms (ClpP1 and ClpP2). So far, all b-lactones were reported to target solely ClpP2 and not ClpP1, raising the question whether monocyclic lactones lack suitable reactivity to interact with the ClpP1 active-site nucleophile.