Nucleus-Encoded Light-Harvesting Chlorophyll a/b Proteins are Imported Normally into Chlorophyll b-free Chloroplasts of Arabidopsis
06-Oct-2012
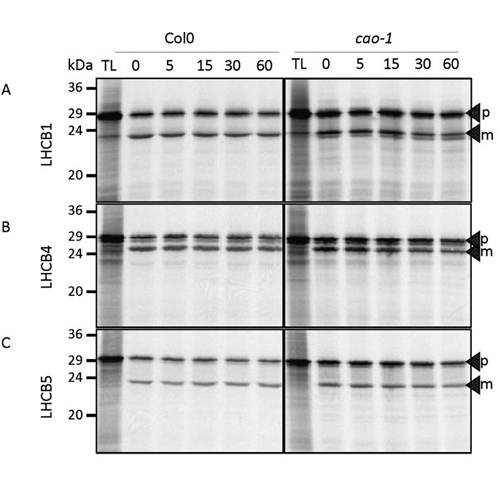
Chloroplast-located proteins which are encoded by the nuclear genome have to be imported from the cytosol into the organelle in a post-translational manner. Among these nuclear-encoded chloroplast proteins are the light-harvesting chlorophyll a/b-binding proteins (LHCPs). After translation in the cytosol, precursor proteins of LHCPs are imported via the TOC/TIC translocase, processed to their mature size to insert into thylakoid membranes were they recruit chlorophylls a and b to form pigment-protein complexes. The translocation of proteins is a highly regulated process which employs several regulators. To analyze whether CAO (chlorophyll a oxigenase) which converts chlorophyll a to chlorophyll b at the inner chloroplast membrane, is one of these regulators we performed import reactions utilizing a homozygous loss-of-function mutant (cao-1). We imported in vitro translated and 35S-labeled precursor proteins of LHCB1, LHCB4 and LHCB5 into chloroplasts isolated from cao-1 and show that import of precursor proteins and their processing to mature forms is not impaired in the mutant. Therefore, regulation of the import machinery cannot be responsible for the decreased steady-state levels of LHC proteins. Regulation does not take place at the transcriptional level either, because Lhcb mRNAs are not down regulated. Additionally, reduced steady-state levels of LHCPs do also not occur due to post-translational turn-over of non-functional LHCPs in chloroplasts. Taken together, our data show that plants in the absence of CAO and therefore devoid of chlorophyll b are not influenced in their import behavior of LHC proteins.