Nucleotide-Free sB-Raf is Preferentially Bound by Hsp90 and Cdc37 In Vitro
09-Sep-2016
Journal of Molecular Biology, Volume 428, Issue 20, Pages 4185–4196, http://dx.doi.org/10.1016/j.jmb.2016.09.002
Journal of Molecular Biology, online article
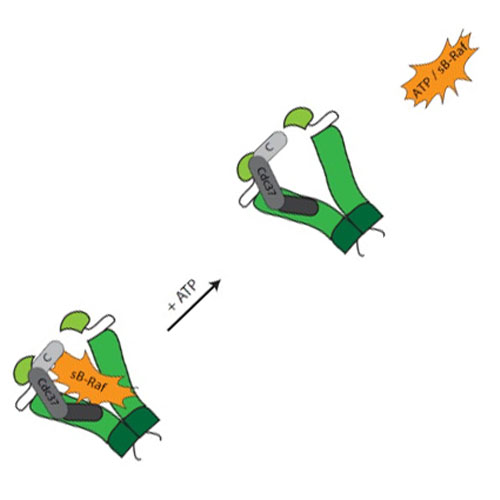
The molecular chaperone Hsp90 and its cofactor Cdc37 are required for the stability of protein kinases in the cellular environment. Upon pharmacological inhibition of Hsp90, the Hsp90-dependent kinases are degraded quickly by the proteasome. Clear physiological evidence for the formation of heterooligomeric complexes between the chaperone system and its kinase clients exist, but the mechanisms of client processing are still enigmatic.
Here, we investigate the interaction of the chaperone system with a stabilized fragment of the Hsp90-dependent protein kinase B-Raf (sB-Raf). sB-Raf is aggregation prone at elevated temperatures. We find that nucleotide binding strongly stabilizes the folded state of sB-Raf and suppresses its aggregation. Also, Cdc37 and Hsp90 in combination can suppress sB-Raf aggregation while forming a ternary complex with the kinase. The presence of nucleotides leads to the dissociation of the kinase from the ternary chaperone complex, implying that the stabilization of the kinase by nucleotides reduces its affinity toward the chaperone machinery. Human Cdc37–Hsp90 complexes can bind to kinase, if the NM domain of the chaperone is present. Nematode Cdc37, which does not require the N-terminal Hsp90 domain for binding, can form a ternary complex with the MC construct of Hsp90, which lacks the aggregation propensity of sB-Raf. Like the full-length complex, this interaction is sensitive to ATP binding to sB-Raf. We thus find that the interaction between sB-Raf and the Hsp90 chaperone system is based on contacts with the M domain of Hsp90, which contributes in forming the ternary complex with CeCdc37 as long as the kinase is not stabilized by nucleotide.