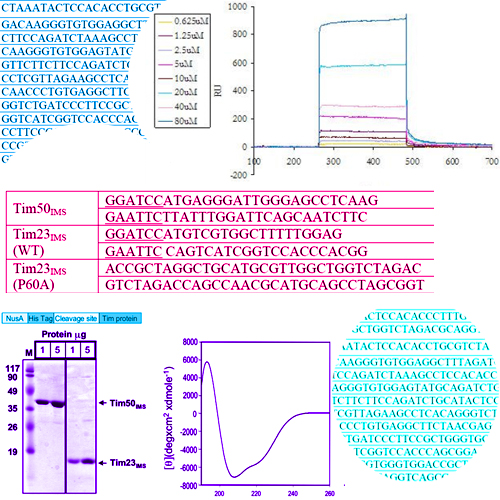
Interaction of tim23 with tim50 is essential for protein translocation by the mitochondrial tim23 complex
18-Nov-2008
The TIM23 complex is the major translocase of the mitochondrial inner membrane responsible for the import of essentially all matrix proteins and a number of inner membrane proteins. Tim23 and Tim50, two essential proteins of the complex, expose conserved domains into the intermembrane space which interact with each other. Here, we describe in vitro reconstitution of this interaction using recombinantly expressed and purified intermembranespace domains of Tim50 and Tim23. We established two independent methods, chemical crosslinking and surface plasmon resonance, to track their interaction. In addition, we identified mutations in Tim23 which abolish its interaction with Tim50 in vitro. These mutations also destabilized the interaction between the two proteins in vivo leading to defective import of preproteins via the TIM23 complex and to cell death at higher temperatures. This is the first study to describe the reconstitution of the Tim50-Tim23 interaction in vitro and to identify specific residues of Tim23 that are vital for the interaction with Tim50.