Characterization of TIC110, a channel-forming protein at the inner envelope membrane of chloroplasts, unveils a response to Ca2+ and a stromal regulatory disulfide bridge
05-Nov-2008
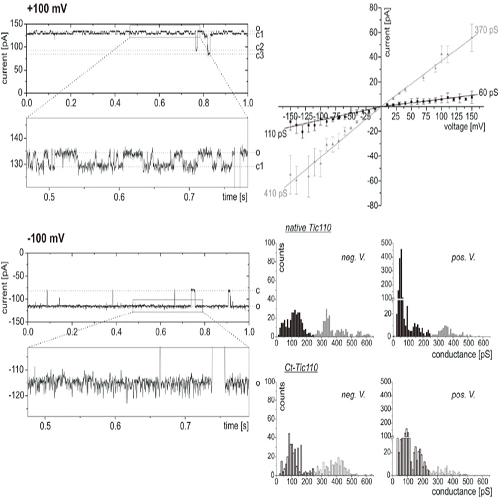
Tic110 has been proposed to be a channel-forming protein at the inner envelope of chloroplasts whose function is essential for the import of proteins synthesized in the cytosol. Sequence features and topology determination experiments presently summarized suggest that Tic110 consists of six transmembrane helices. Its topology has been mapped by limited proteolysis experiments in combination with mass spectrometric determinations and cysteine modification analysis. Two hydrophobic transmembrane helices located in the N-terminus serve as a signal for the localization of the protein to the membrane as shown previously. The other amphipathic transmembrane helices are located in the region comprised of residues 92-959 in the pea sequence. This results in two regions in the intermembrane space localized to form supercomplexes with the TOC machinery and to receive the transit peptide of preproteins. A large region also resides in the stroma for interaction with proteins such as molecular chaperones. In addition to characterizing the topology of Tic110, we show that Ca2+ has a dramatic effect on channel activity in vitro and that the protein has a redox-active disulfide with the potential to interact with stromal thioredoxin.