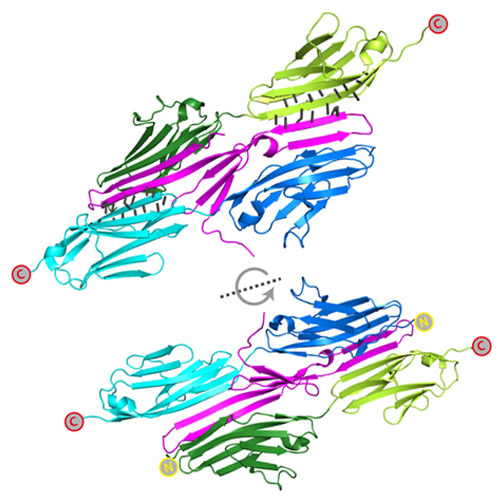
The titin-telethonin complex is a directed, superstable molecular bond in the muscle Z-disk
21-Jul-2009
PNAS, 2009, doi: 10.1073/pnas.0902312106, vol. 106 no. 32 13307-133310 , published on 09.11.2009
PNAS, online article
PNAS, online article
Mechanical stability of bonds and protein interactions has recently become accessible through single molecule mechanical experiments. So far, mechanical information about molecular bond mechanics has been largely limited to a single direction of force application. However, mechanical force acts as a vector in space and hence mechanical stability should depend on the direction of force application. In skeletal muscle, the giant protein titin is anchored in the Z-disk by telethonin. Much of the structural integrity of the Z-disk hinges upon the titin-telethonin bond. In this paper we show that the complex between the muscle proteins titin and telethonin forms a highly directed molecular bond. It is designed to resist ultra-high forces if they are applied in the direction along which it is loaded under physiological conditions, while it breaks easily along other directions. Highly directed molecular bonds match in an ideal way the requirements of tissues subject to mechanical stress.