Mechanical Stability of the Antibody Domain CH3 Homodimer in Different Oxidation States
10-Sep-2013
J. Am. Chem. Soc., 2013, DOI: 10.1021/ja405076j, 135 (40), pp 15085–15091 published on 10.09.2013
J. Am. Chem. Soc., online article
J. Am. Chem. Soc., online article
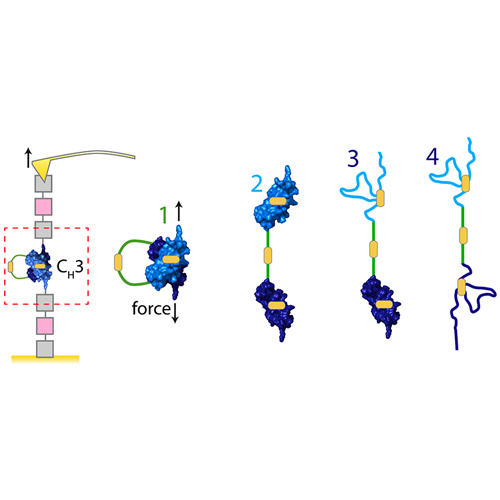
The CH3 homodimer at the C-terminal end of the antibody heavy chain is the key noncovalent interaction stabilizing antibody proteins. Here, we use single-molecule force spectroscopy to investigate the dissociation mechanics of CH3 as a proxy for antibody mechanical stability. We find the CH3 homodimer to be a highly stable complex, and its dissociation force of >150 pN at a loading rate of ≈5500 pN/s exceeds the stability of most protein–protein interactions studied to date. Separated CH3 monomers, on the other hand, are mechanically labile and only short-lived. Each CH3 monomer contains a conserved buried disulfide bridge, and we find that the successive reduction of one or both disulfide bridges in the dimer results in a stepwise decrease of the dissociation force. This suggests a structural role of the disulfide bridges helping to mold the high-affinity domain–domain interface, even though they are neither required for nor directly involved in dimerization. Taken together, our results set a limit on how much force a single antibody can bear and reveal the CH3 homodimer as a mechanical fastener that prevents antibody dissociation.