Comparing a Photoinduced Pericyclic Ring Opening and Closure: Differences in the Excited State Pathways
14-Jun-2007
Journal of the American Chemical Society, 2007, 129, 8577-84 published on 14.06.2007
Journal of the American Chemical Society, online article
Journal of the American Chemical Society, online article
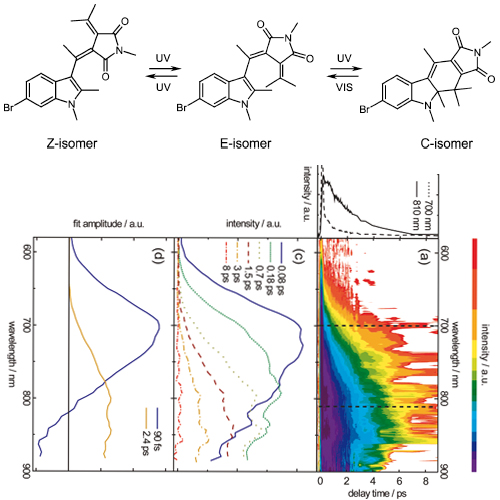
The photochromicity of fulgimides rests on the existence of open (E) and closed ring (C) isomers. As predicted by the Woodward-Hoffmann rules both isomers can photochemically be interconverted. This interconversion has been studied by femtosecond fluorescence and transient absorption spectroscopy. For either direction (E toC cyclization and C to E cycloreversion) a biphasic fluorescence decay on the 0.1-1 ps time scale is observed. The longer time constants of the decays equal the formation times of the photoproducts. The time constants retrieved (0.06 and 0.4 ps for E toC, 0.09 and 2.4 ps for C to E) and the associated spectral signatures differ substantially. This indicates that no common excited-state pathway for the two directions exists, as one would infer from a simple Woodward-Hoffmann consideration. These findings support recent quantum dynamic calculations on the excited-state topology of fulgimides.